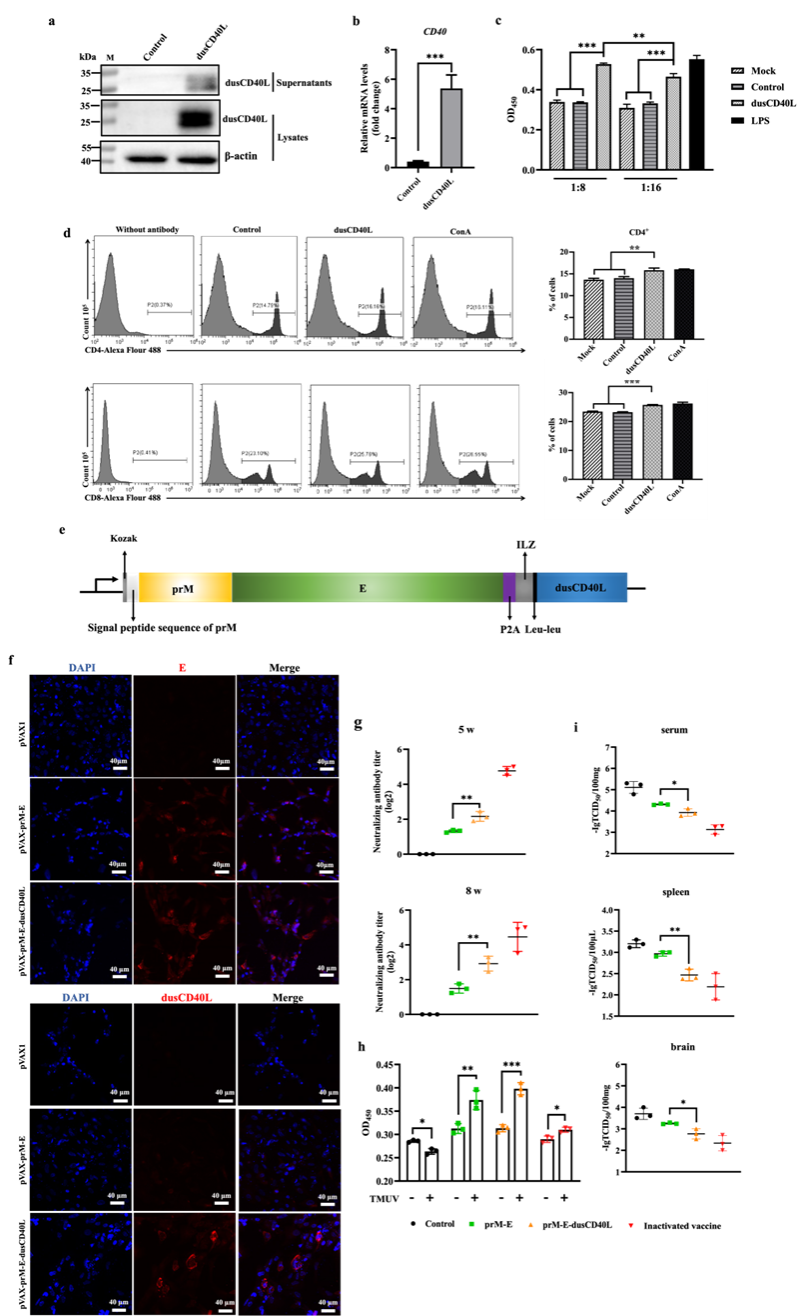
Numerous viruses have the potential for transmission from animals to humans, including but not limited to the influenza virus (IV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Japanese encephalitis virus (JEV), and dengue virus (DENV). Within the spectrum of zoonotic pathogens, avian species often serve as either intermediate or natural hosts. Bird trade and human movements may be a possible mode for flaviviruses spread. Vaccination is a pivotal strategy for prevention and containment of virus transmission. DNA vaccines, characterized by their safety profile, ease of production, and transportability, have emerged as promising candidates for the prevention of emerging infectious diseases in birds. Immunization with a DNA vaccine yielded limited protection against avian pathogen infection. To improve its immunogenicity, the extracellular domain of duck-derived CD40L (designated as dusCD40L) was employed as a bio-adjuvant. Our findings unequivocally established the evolutionary conservation of dusCD40L across avian species. Notably, dusCD40L exhibited a compelling capacity to elicit robust immune responses from both B and T lymphocytes. Furthermore, when employed as an adjuvant, dusCD40L demonstrated a remarkable capacity to significantly augment the titers of neutralizing antibodies and the TMUV-specific T cell responses post a DNA vaccine encoding the prM-E region of an avian flavivirus (Tembusu virus, TMUV) immunization. Moreover, dusCD40L could strengthen virus clearance of the prM-E DNA vaccine in ducks post-TMUV challenge. This research study presents a highly effective adjuvant for advancing the development of DNA vaccines targeting TMUV in avian hosts. Additionally, it underscores the pivotal role of duCD40L as a potent adjuvant in the context of vaccines designed to combat zoonotic infections in avian species. https://doi.org/10.1038/s41541-024-00926-9.