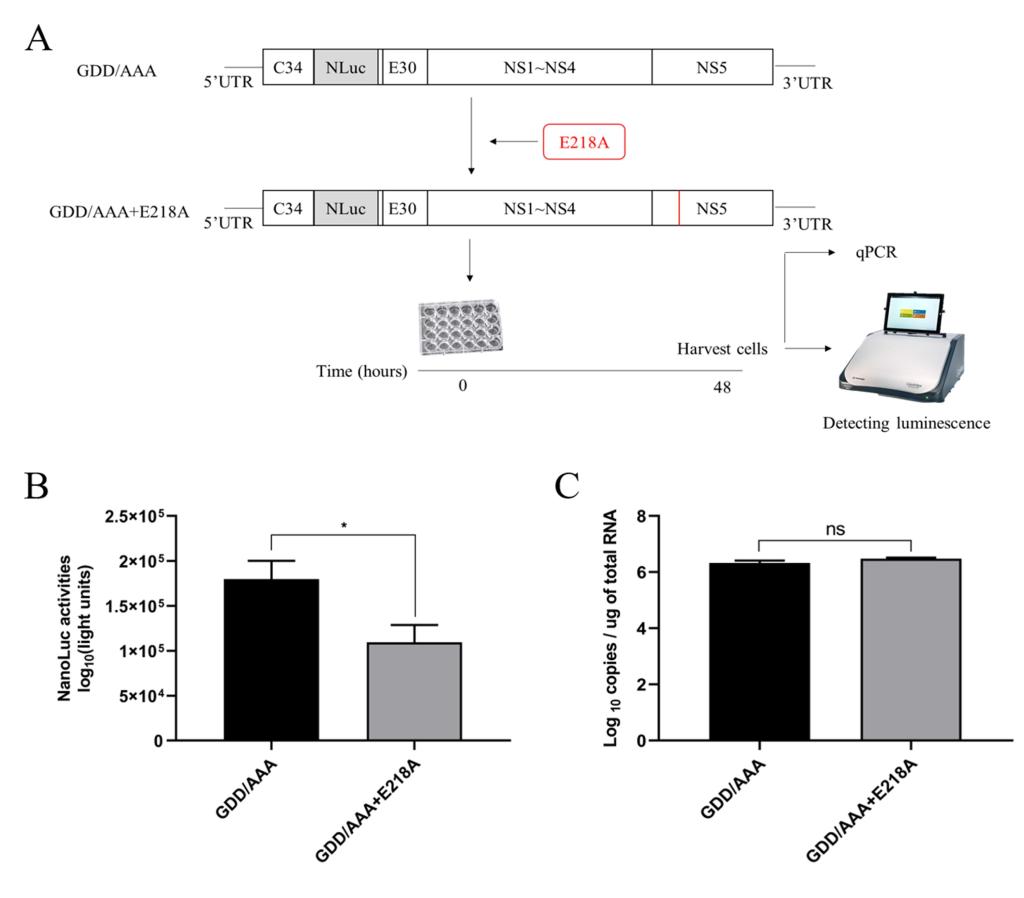
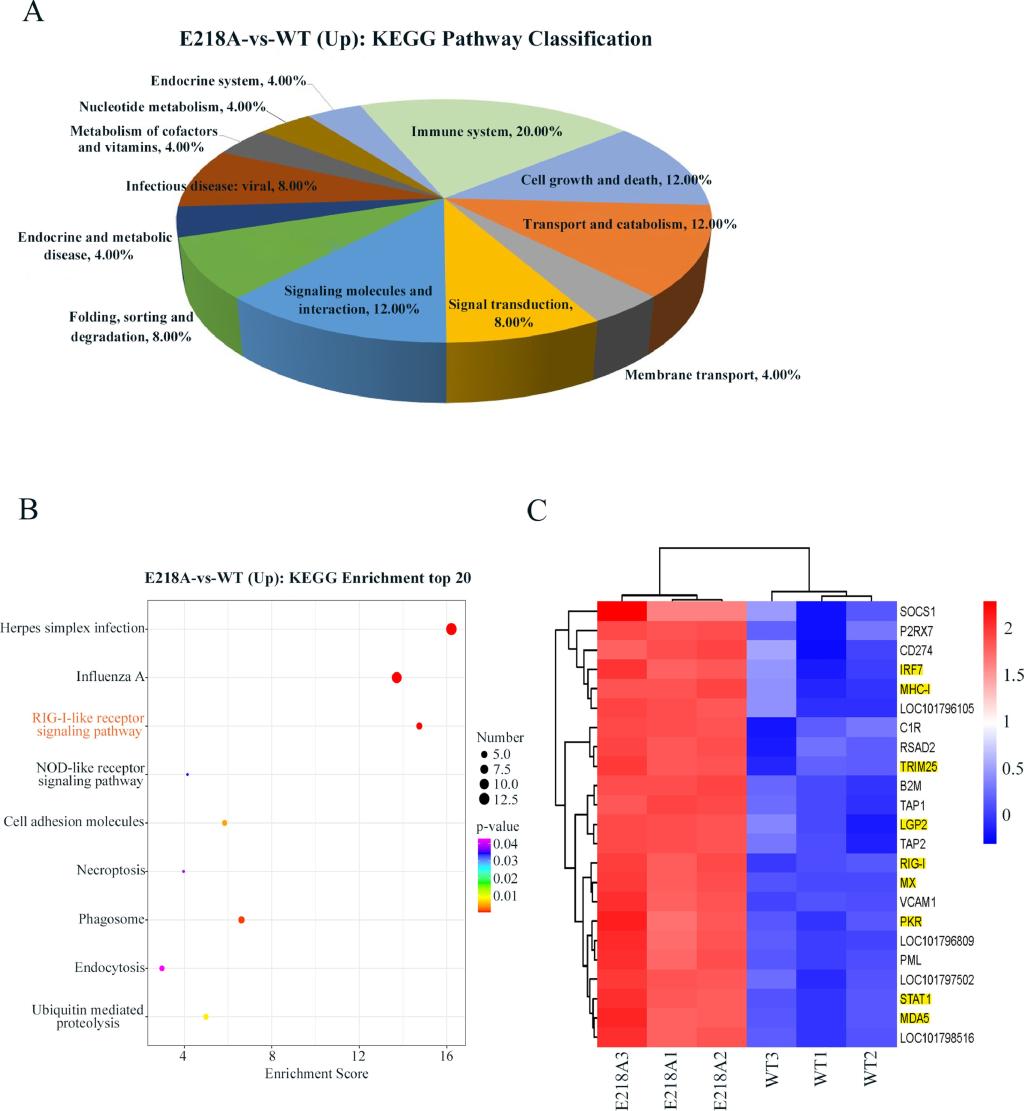
The N-terminus of duck Tembusu virus (DTMUV) NS5 protein contains the conserved methyltransferase (MTase) domain, and E218 is one of the active sites of MTase. It has been shown that the DTMUV E218A mutation disables the 2’-O-MTase activity of the virus and significantly reduces the N-7-MTase activity. This study was conducted based on the possibility that reduced MTase activity may affect the viral life cycle and host immune response. The results showed that E218A mutation inhibited viral replication and translation, and may activate host RIG-I like receptor signaling to induce the production of antiviral proteins, ultimately leading to the reduction of viral proliferation. This study elucidated the function of DTMUV MTase in virus life cycle and escape from host innate immune response, and provided new information for understanding the pathogenic mechanism of DTMUV. The study was published in the
and titled with ‘The substitution at residue 218 of the NS5 protein methyltransferase domain of Tembusu virus impairs viral replication and translation and may triggers RIG-I-like receptor signaling’. 2022, 101:102017,
https://doi.org/10.1016/j.psj.2022.102017