Introduction
Waterfowl parvoviruses can cause different disease conditions depending on the species and the age of affected waterfowl. The highly contagious fatal disease affecting young geese (Fig 1) and Muscovy ducks, caused by goose parvovirus (GPV) was described under different names depending on the major clinical–pathological features that were observed. Goose Parvorirus (GPV) was clade to Anseriform dependoparvovirus 1, as the members of Dependoparvovirus genus of the Parvovirinae subfamily within the Parvoviridae family. GPV, which is fetal for gosling and can cause enteritis and diarrhoea, is the etiological agent of Derzsy’s disease. GPV is a single-stranded DNA virus without envelop protein and its complete genome is about 5.1 kb in length, which contains two major open reading frames (ORF) and the inverted terminal repeats at the both genomic terminus. The left ORF encodes the non-structural protein required for both replication of viral genome and regulation of capsid gene expression, and the right ORF encodes the structural protein composed of three capsid protein which have common region of C-terminus.
A syndrome associated with parvovirus infection causing short beak and dwarfism syndrome, also called as beak atrophy and dwarfism syndrome (Fig 2), in Muscovy, mule, and Pekin ducks was caused by a Novel Goose Parvovirus (NGPV). It is a moderately pathogenic GPV-related parvovirus, has been reported in commercial Cherry Valley duck flocks and mule duck flocks in China since 2015. A strain of NGPV isolated from clinical samples, named as NGPV-SC16 (the accession number KY679174), was studied in our research center.

Figure 1Enteric form of natural GPV infected cherry valley duck. Haemorrhagicnecrotic enteritis with pseudomembrane formation in the small intestine.

Figure 2Clinical symptom of naturally NGPV infected cherry valley duck (6 weeks old), showing beak atrophy and dwarfism syndrome, deformed legs (where red arrow is pointing).
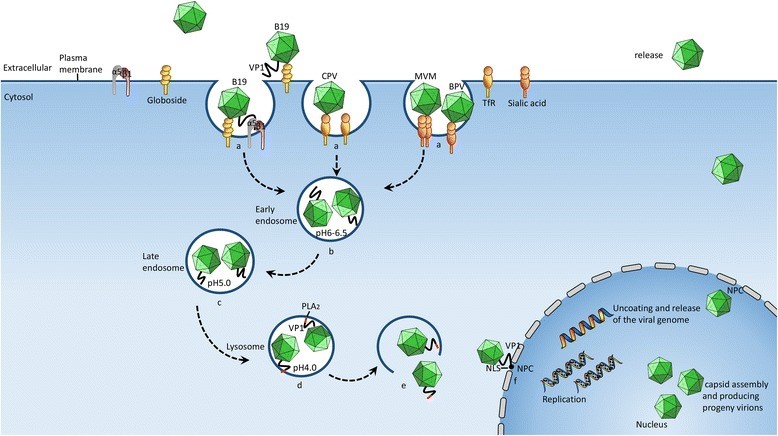
Figure 3 A schematic of the parvovirus infection process, mediated by the Clathrin-dependent endocytic pathway. The internalization of the virus is mediated by the endocytosis pathway, primarily through the following steps.
Current research interests
1. The evolution and mutation of GPV, NGPV, and MDPV.
2. The pathogenesis study of GPV, NGPV, and MDPV.
3. The immune interaction between the host and GPV and NGPV.
Goose Astrovirus
Introduction
Goose astrovirus, belonging to the family Astrovirus and genus Avian Astrovirus, is a small, non-enveloped, single-strand positive-sense RNA virus. Goose astrovirus causes a disease characterized by severe visceral gout and articular gout (Fig 1-3). Isolates originated from avian species, such as turkey, chickens, ducks, and other birds are classified into the genus Astrovirus. Since 2016, goose astrovirus has broken out among Chinese goslings, causing at most 50% death of goslings. Until now, at least 80 strains of Goose astrovirus genome have been reported and conserved. Their genomes are 6.8-7.9 kb in length, consisting of a 5′-untranslated region (UTR), three open reading frames (ORFs), a 3′-UTR and a poly (A) tail. The high degree of genetic diversity among astrovirus and their recombination potential signify their capacity to cause a broad spectrum of diseases in multiple host species. The core antigen of goose astrovirus is Cap protein. Significantly, goose astrovirus can vertically spread from breeding geese to goslings and effectively infect Pekin-ducks and Muscovy ducks across species barriers, posing a greater threat to the development of waterfowl industry. Although goose astrovirus causes serious economic losses, the pathogenic mechanisms and the potential antiviral targets of goose astrovirus are largely unknown.
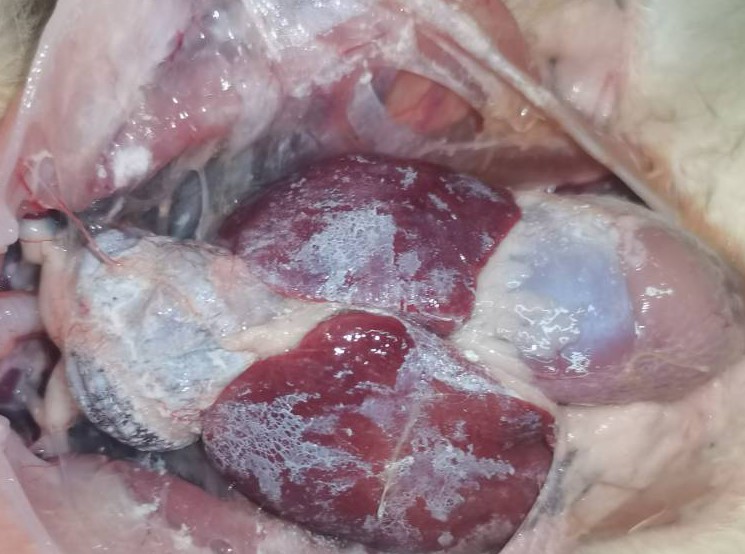
Figure 1White urate deposition in the heart and liver of goose astrovirus infected goslings
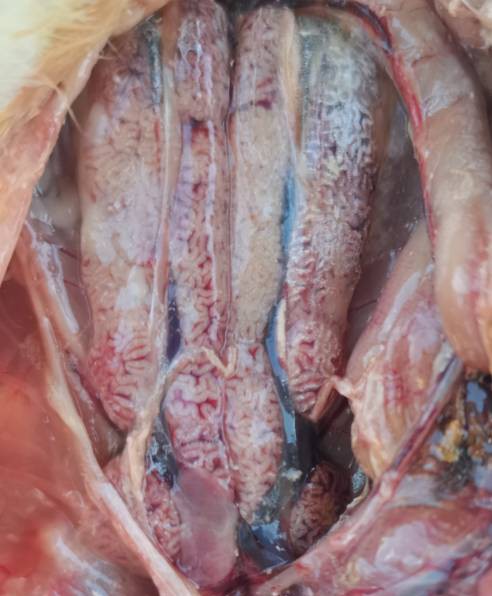
Figure 2The kidney of goose astrovirus infected goslings Swells and bleeds to form piebald spots; White urate deposits in spleen and ureter.
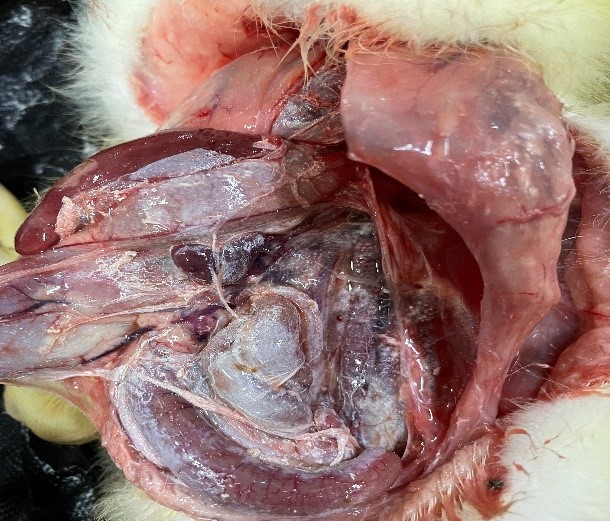
Figure 3 Large amount of white urate deposits in the liver and spleen of goose astrovirus infected goslings
Current research interests
1. Developing the detection methods of Goose astrovirus and its coinfection with other avian viruses.
2. Epidemic, evolution and variation of Goose astrovirus.
3. Pathogenic mechanisms of Goose astrovirus.
4. Novel vaccine development.
Selected publications (*corresponding author)
1. Shun Chen*, Peng Liu, Yu He, Chao Yang, Mingshu Wang, Renyong Jia, Dekang Zhu, Mafeng Liu, Qiao Yang, Ying Wu, Anchun Cheng*. The 164K 165K and 167K residuesin 160YPVVKKPKLTEE171 are required for the nuclear import of gooseparvovirus VP1. Virology, 2018 Mar 22; 519(2018)17-22
2. Peng Liu, Shun Chen*, Mingshu Wang, Anchun Cheng*. The role of nuclear localization signal in parvovirus life cycle. Virology Journal, 2017 April 14; 14(1): 80.
3. Shun Chen, Anqi Wang, Lipei Sun, Fei Liu, Mingshu Wang, Renyong Jia, Dekang Zhu, Mafeng Liu, Qiao Yang, Ying Wu, Kunfeng Sun, Xiaoyue Chen, Anchun Cheng*. Immune-Related Gene Expression Patterns in GPV- or H9N2-Infected Goose Spleens. International Journal of Molecular Sciences, 2016 Dec 1; 17(12): 1990.
4. Hao Zhou, Shun Chen*, Mingshu Wang, Renyong Jia, Dekang Zhu, Mafeng Liu, Fei Liu, Qiao Yang, Ying Wu, Kunfeng Sun, Xiaoyue Chen, Bo Jing & Anchun Cheng*. Antigen distribution of TMUV and GPV are coincident with the expression profiles of CD8α-positive cells and goose IFNγ. Scientific Reports, 2016 May 6; 6: 25545.
5. Mengyu Tu, Fei Liu, Shun Chen*, Mingshu Wang and Anchun Cheng*. Role of capsid proteins in parvoviruses infection. Virology Journal, 2015 Aug 4; 12: 114.
6. Jinyue Zhang, Peng Liu, Yuanyuan Wu, Mingshu Wang, Renyong Jia, Dekang Zhu, Mafeng Liu, Qiao Yang, Ying Wu, Xinxin Zhao, Shaqiu Zhang, Yunya Liu, Ling Zhang, Yanling Yu, Yu You, Shun Chen* and Anchun Cheng. Growth characteristics of the novel goose parvovirus SD15 strain in vitro[J]. BMC Vet Res. 2019 Feb 19;15(1):63.
7. Peng Liu, Liqin Yang, Jingyue Zhang, Tao Wang, Yuanyuan Wu, Mingshu Wang, Renyong Jia, Dekang Zhu, Mafeng Liu, Xinxin Zhao, Qiao Yang, Ying Wu, Shaqiu Zhang, Yunya Liu, Yanling Yu, Ling Zhang, Leichang Pan, Shun Chen* and Anchun Cheng*. The 164 K, 165 K, and 167 K residues of VP1 are vital for goose parvovirus proliferation in GEFs based on PCR-based reverse genetics system[J]. Virology Journal volume, Nov14 2019, 136 (2019).
8. Peng Liu, Jinyue Zhang, Shun Chen*, Mingshu Wang, Anchun Cheng. Genome Sequence of a Goose Parvovirus Strain Isolated from an Ill Goose in China[J]. Genome Announcements, 2017 Apr 27; 5(17): e00227-17.
9. Zhiqi Ge, Shun Chen*, Mingshu Wang, Anchun Cheng. Complete Genome Sequence of a Novel Goose Parvovirus Isolated in Sichuan Province, China, in 2016[J]. Genome Announcements, 2017 Jun 8; 5(23): e00428-17.
10. Dawei Xu, Chuanfeng Li, Guangqing Liu, Zongyan Chen* and Renyong Jia*. Generation and evaluation of a recombinant goose origin Newcastle disease virus expressing Cap protein of goose origin avastrovirus as a bivalent vaccine in goslings[J]. Poultry Science, 2019 April 14, 0:1–7,10(98)4426-4432, doi: org/10.3382/ps/pez255.
Contact Details
Shun Chen, Ph.D, Professor
Institute of Preventive Veterinary Medicine
Sichuan Agricultural University
No. 211, Huimin Road, Chengdu, Sichuan 611130, China
Email:
shunchen@sicau.edu.cn
sophia_cs@163.com
Duck Circovirus
Duck circovirus (DuCV) is a member of the genus Circovirus, family Circoviridae. DuCV was originally reported in two female 6-week-old Mulard ducks from a German farm in 2003. Currently, DuCV is prevalent in China. It causes stunting and feather abnormalities. Lymphoid depletion predisposes the host to immunosuppression, and disease progression is further complicated by co-infections with other bacterial and viral pathogens. So far, the precise pathogenic mechanism of the virus is unclear. In 2013, a DuCV (GenBank: JX499186) was isolated from Muscovy ducks in Sichuan. According to its genome, the ELISA based on capsid protein, infectious DNA clones were development to study the pathogenic mechanism of DuCV.
Current research interests
1. Epidemiology investigation of DuCV in waterfowl.
2. Mechanism of DuCV induced apoptosis.
3. Vaccine development.
Duck Reovirus
Duck reovirus (DRV) is belong to the genus Orthoreovirus, family Reoviridae. DRV was introduced to China in 1997. DRV mainly infects Muscovy duck with arthritis/tenosynovitis, and it was nonpathogenic for Peking ducks. Since 2011, isolates that can cause death in vary ducks have increasingly been reported. These isolates named novel duck reovirus (NDRV). In 2020, a duck reovirus (GenBank submission ID: 2604364) was isolated from swan in Southwest of China. It mainly causes white necrotic spots in liver and spleen of Peking duck, and even death. The reasons for the expanded host range and increased virulence have not yet been declared.
Current research interests
1. Molecular evolution analysis of NDRV.
2. The interaction between host and NDRV.
3. Vaccine development.
Selected Publications
1. Yang Cui, Xu Yu, Jia Ren-yong*, Liu Si-yang, Wang Ming-shu, Zhu De-kang, Chen Shun, Liu Ma-feng, Zhao Xin-xin, Sun Kun-feng, Jing Bo, Yin Zhong-qiong, Cheng An-chun. The codon-optimized capsid gene of duck circovirus can be highly expressed in yeast and self-assemble into virus-like particles[J]. Journal of Integrative Agriculture, 2017, 16(7): 1601-1608.
2. Cui Yang, Yu Xu, Renyong Jia*, Pengfei Li, Lei Zhang, Mingshu Wang, Dekang Zhu, Shun Chen, Mafeng Liu, Zhongqiong Yin, Anchun Cheng. Prokaryotic expression of a codon-optimized capsid gene from duck circovirus and its application to an indirect ELISA[J]. Journal of Virological Methods, 2017, 247: 1-5.
3. Pengfei Li, Zhilong Zhang, Renyong Jia*, Sai Mao, Mingshu Wang, Ruiling Jia, Mafeng Liu, Dekang Zhu, Shun Chen, Kunfeng Sun, Zhongqiong Yin, Xiaoyue Chen and Anchun Cheng. Rescue of a duck circovirus from an infectious DNA clone in ducklings[J]. Virology Journal, 2015, 12: 82.
4. Yanyan Lu, Renyong Jia*, Zhilong Zhang, Mingshu Wang, Yu Xu, Dekang Zhu, Shun Chen, Mafeng Liu, Zhongqiong Yin, Xiaoyue Chen, Anchun Cheng. In vitro expression and development of indirect ELISA for Capsid protein of duck circovirus without nuclear localization signal[J]. International Journal of Clinical and Experimental Pathology, 2014, 7(8): 4938-4944.
5. Zhilong Zhang, Renyong Jia*, Yanyan Lu, Mingshu Wang, Dekang Zhu, Shun Chen, Zhongqiong Yin, Xiaoyue Chen, Anchun Cheng. Identification, genotyping, and molecular evolution analysis of duck circovirus[J]. Gene, 2013, 529(2): 288-295.
6. Zhilong Zhang, Renyong Jia*, Mingshu Wang, Yanyan Lu, Dekang Zhu, Shun Chen, Zhongqiong Yin, Yin Wang, Xiaoyue Chen, Anchun Cheng. Complete Genome Sequence of the Novel Duck Circovirus Strain GH01 from Southwestern China[J]. Genome Announcement, 2013, 1(1): e00166-12.
Contact Details
Renyong Jia, Ph.D, Professor
Institute of Preventive Veterinary Medicine
Sichuan Agricultural University
No. 211, Huimin Road, Chengdu, Sichuan 611130, China
Email:
jiary@sicau.edu.cn
Juan Huang, Ph.D, Lecturer
Institute of Preventive Veterinary Medicine
Sichuan Agricultural University
No. 211, Huimin Road, Chengdu, Sichuan 611130, China
Email:
huangjuan@sicau.edu.cn;
huangjuan610@163.com

